Addition of Hydrogen Halides to Alkenes Mechanism
Preparation of Hydrocarbons Alkenes. As shown in the following figure a hydrogen ion catalyzes the Markovnikovs addition.
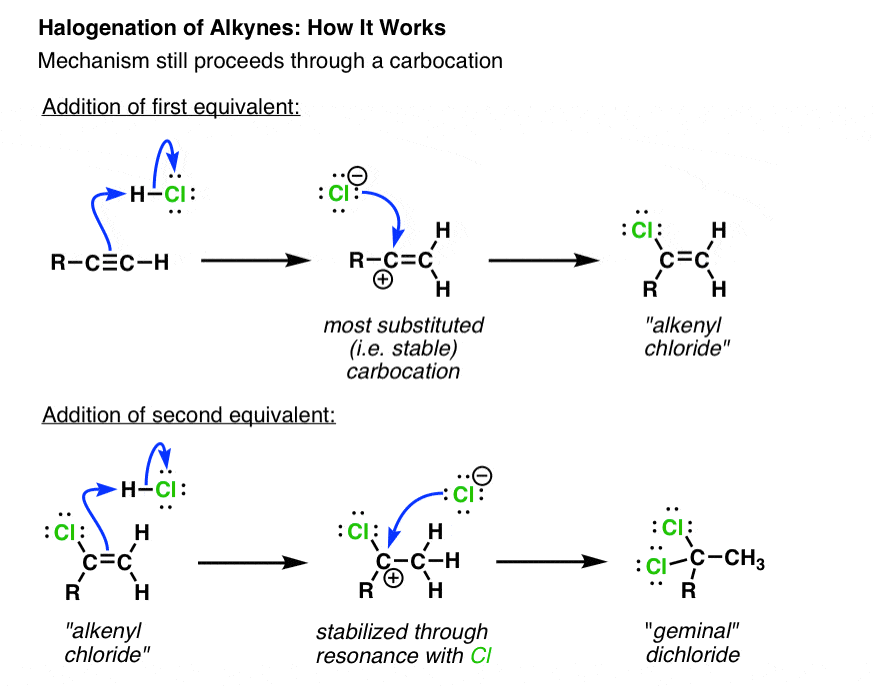
Addition Of Hydrogen Halides Hcl Hbr Hi To Alkynes Hydrohalogenation
Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations the most.
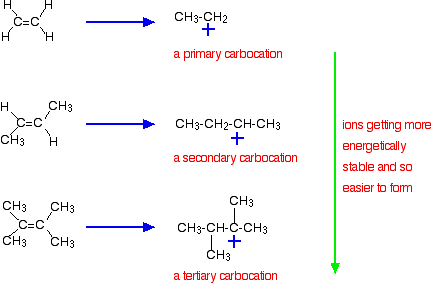
. Most of the reactions involving the preparation of alkenes involve elimination process. 3 CH 3 3 C-Br CN CH 3 2 CCH 2 Br HCN We know that t-butyl bromide is not expected to react by an S N 2 mechanism. In four textbooks where SOCl 2 is mentioned the reaction is shown as proceeding through an S N 2 mechanism.
However often the two are used interchangeably because the. The S N 2 doesnt happen for secondary alcohols. The antiMarkovnikovs addition results from a hydroborationoxidation reaction.
Organoboron chemistry or organoborane chemistry is the chemistry of these compounds. Organic Chemistry Study Materials Practice Problems Summary Sheet Guides Multiple-Choice Quizzes. All these eliminations are β- eliminations.
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. 1212 Structure of the Carbonyl. Theres no warning sign saying wait.
You will find it - Its all here. The following Diels-Alder cycloaddition was recently performed by Mukherjee et al. We have not yet considered the factors that influence elimination reactions such as example 3 in the group presented at the beginning of this section.
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH 3 for example trialkyl boranes. The elements of water can be added to the doublebonded carbons of an alkene in either a Markovnikovs or an antiMarkovnikovs manner. In addition the oxygen atom also has two non bonding electron pairs.
Thus the carbonyl carbon and the three atoms attached to it lie in the same plane and the π-electron cloud is above and below this plane. Furthermore the ethanol solvent is not. Only one textbook in this admittedly incomplete sample mentions the S N i mechanism at all.
The bond angles are approximately 120 as expected of a trigonal coplanar structure Figure 121. There are 3 mechanisms suggested for the elimination reactions. It is a subset of reactions that very closely resembles the insertion reactions and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products.
C n H 2n. CHCl COEt ACN CoEt AcOEt CN -78C 8h 25 75 When cyclopentadiene is added in excess the reaction is pseudo-1 order with respect to the fumaric nitrile ester.
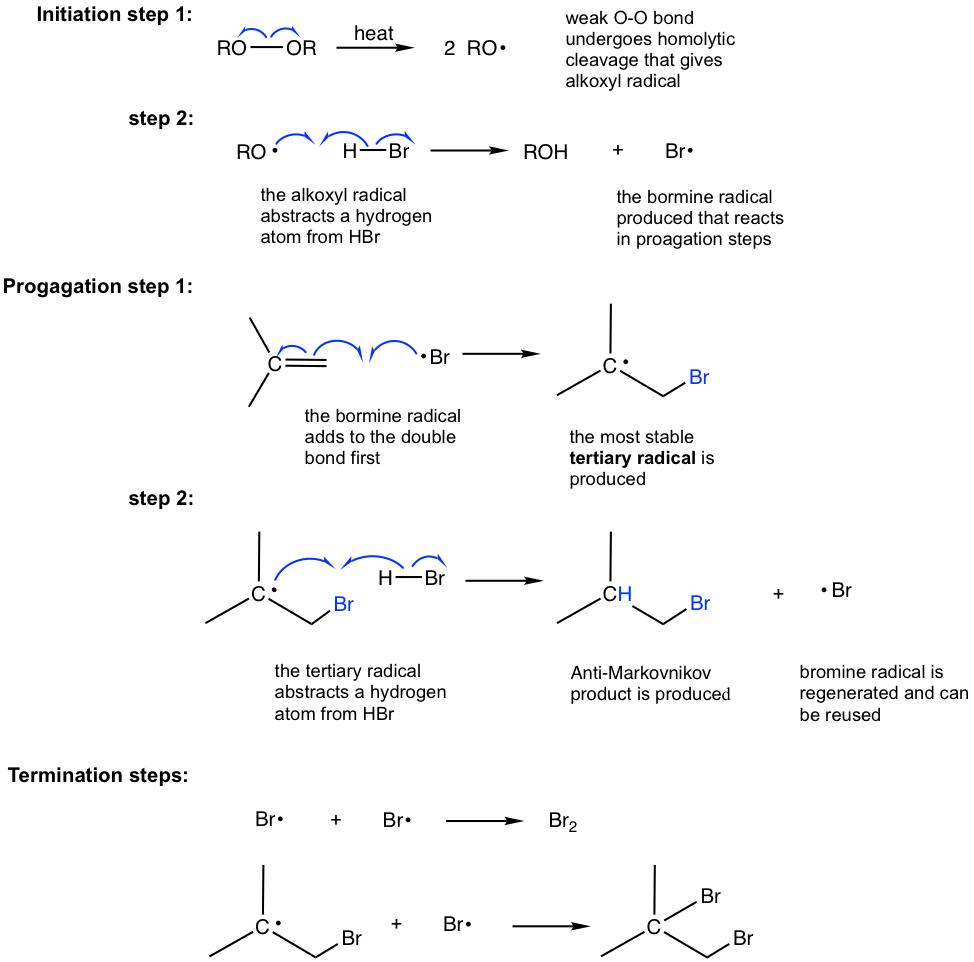
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I

Electrophilic Addition Of Hydrogen Halides To Alkenes Youtube

Regioselectivity Of Hydrogen Halide Addition Markovnikov S Rule Youtube

9 2 Addition Of Hydrogen Halides To Symmetrical Alkenes Chemistry Libretexts

Electrophilic Addition Reactions Of Alkenes Mcc Organic Chemistry
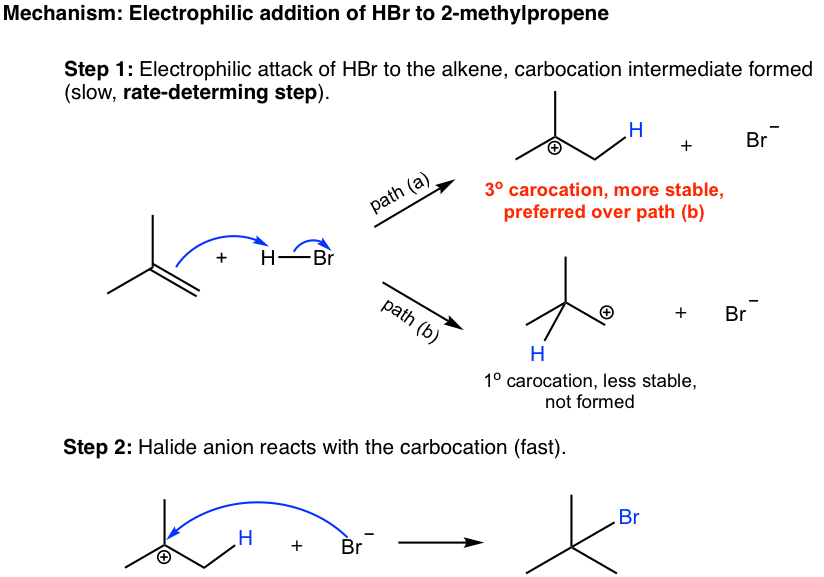
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I
0 Response to "Addition of Hydrogen Halides to Alkenes Mechanism"
Post a Comment